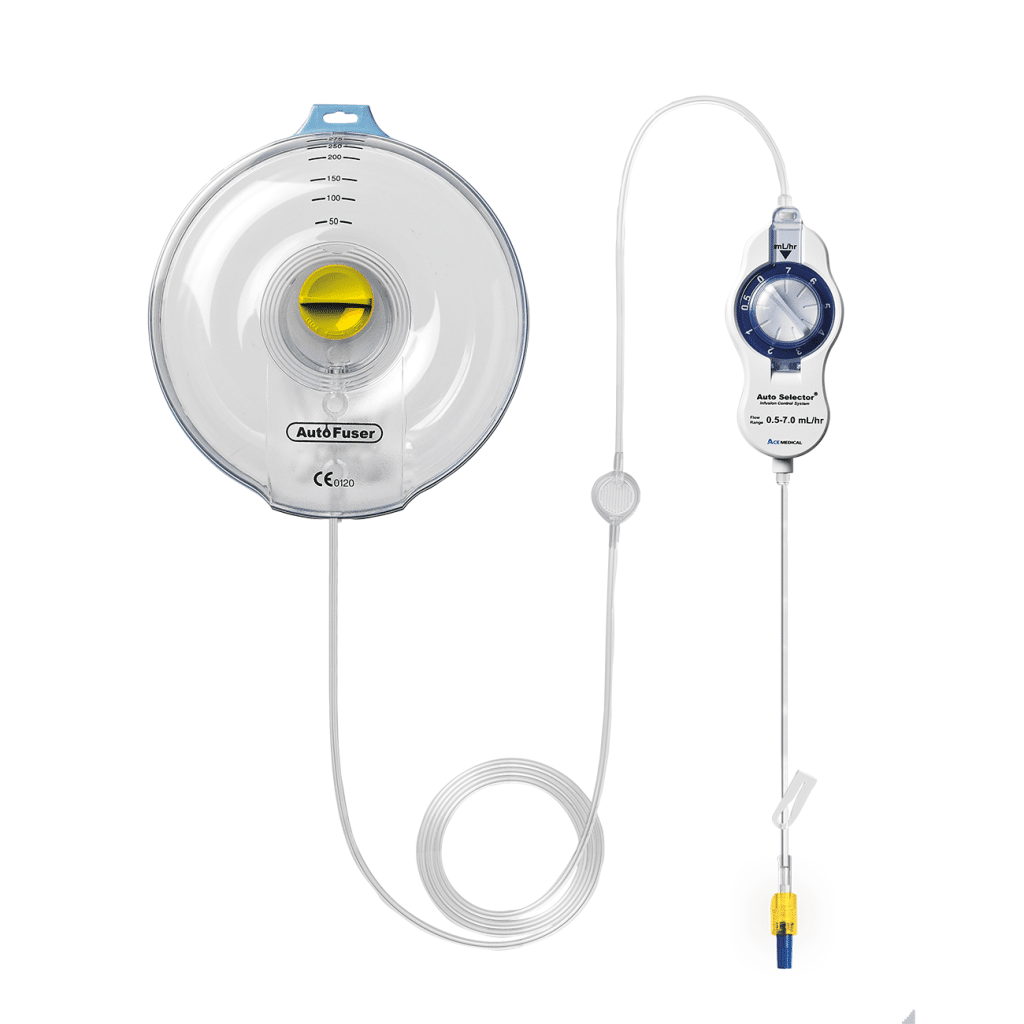
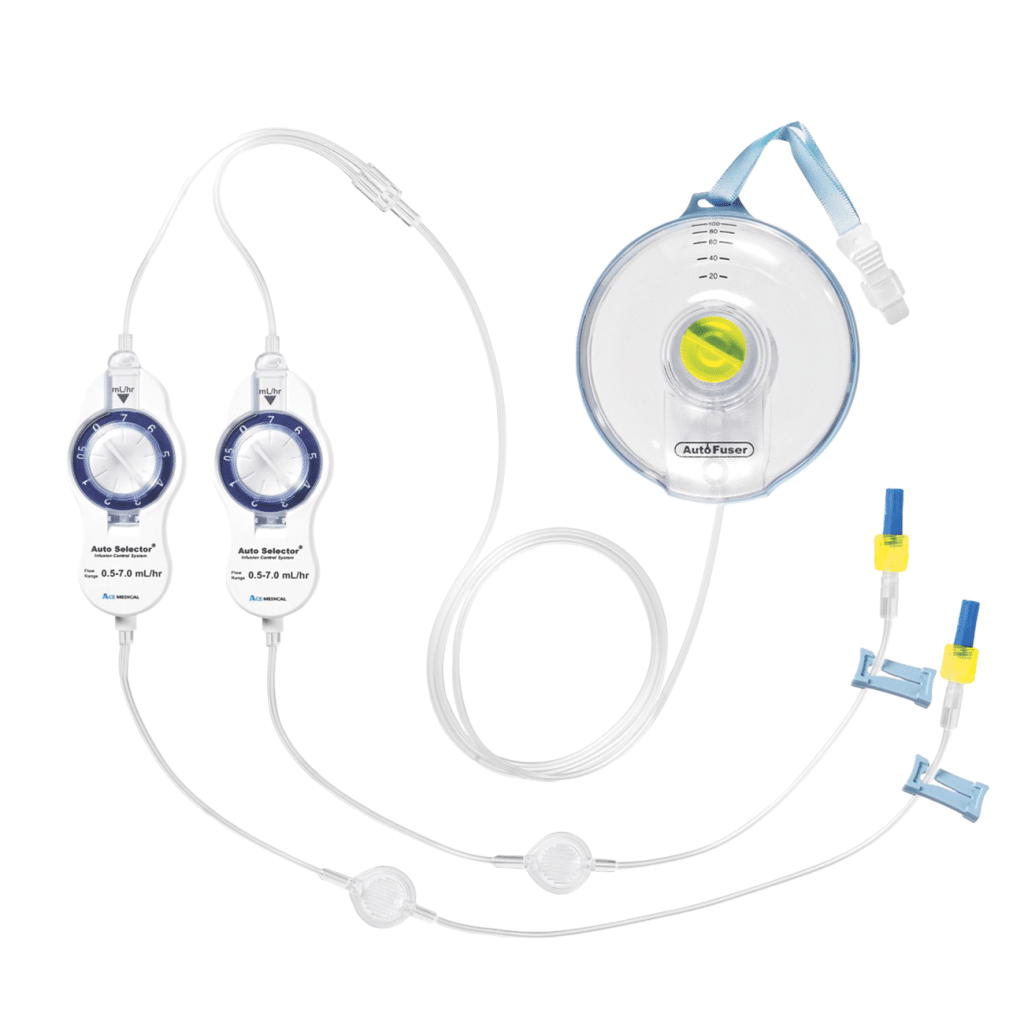
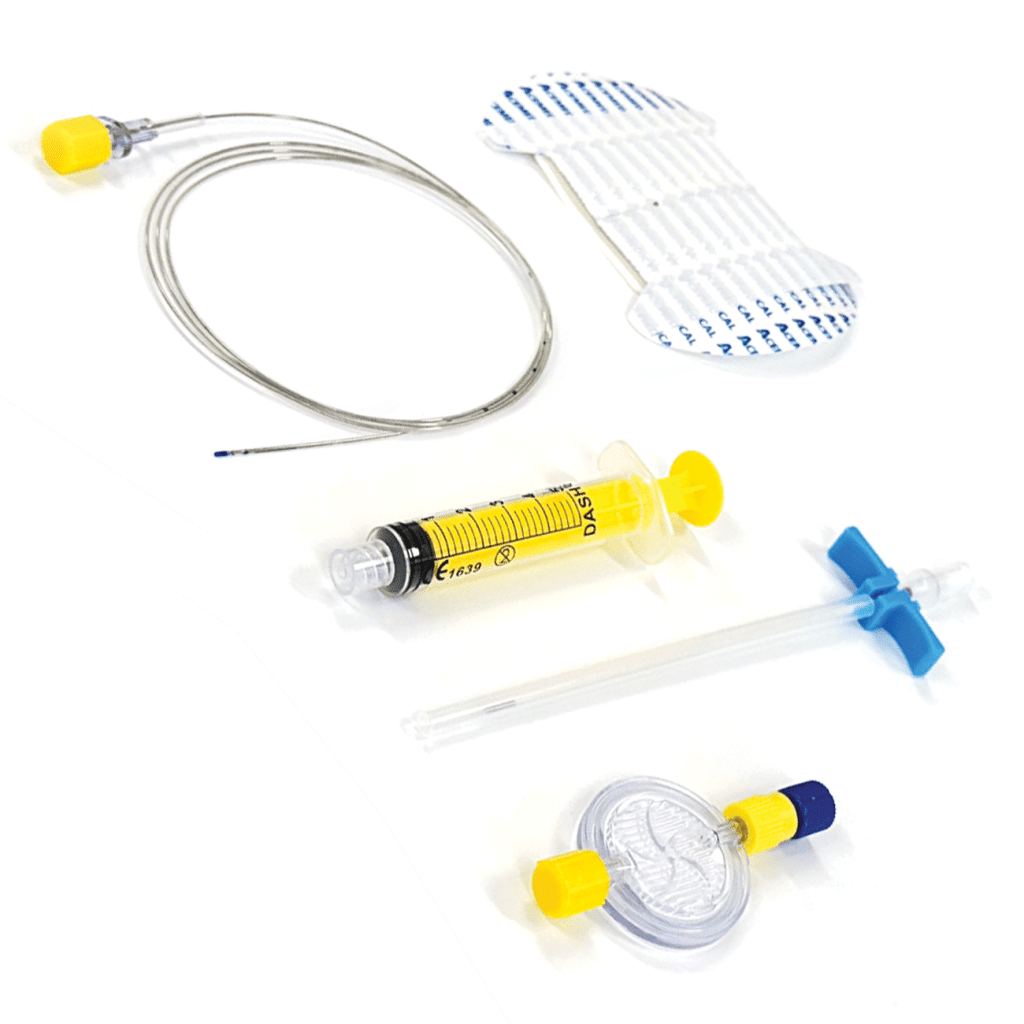
Are you NRFit ready?

it’s crucial to prepare
New NRFit® Connector
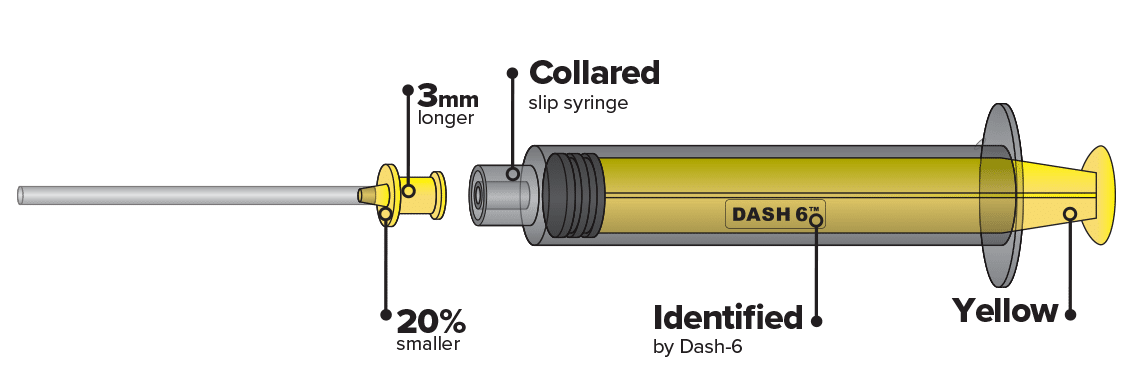
All needle and component connectors are changing to NRFit. These design features make it unlikely that a medical device intended for neuraxial applications will fit together with devices intended for other clinical applications such as IV or enteral.
Products
Identifying NRFit® Devices
Our solution in preventing wrong route administration

- NRFit® devices comply with ISO 80369-6 and feature the NRFit logo on packaging and, when possible, on the device.
- NRFit® and non-NRFit connectors are incompatible, ensuring safety.
- GBUK NRFit devices are branded DASH-6.
- Yellow color coding is commonly used, although not mandated by ISO 80369-6.

Frequently Asked Questions
What is a small-bore connector
A small-bore connector is a connector with an inner diameter of less than 8.5mm used to connect medical devices, components, and accessories for the purposes of delivering fluids or gases.
What changes are coming to small-bore connectors
The International Organization for Standardisation (ISO) has developed a series of standards for small-bore connectors, known as the ISO 80369 series. These standards provide design and performance specifications for a range of connectors used in different medical device applications, such as 80369-3 enteral (ENFit), 80369-6 neuraxial (NRFit), and 80369-7 intravascular and hypodermic (Luer). These standards will help reduce the risks of misconnections between different applications.
Will new neuraxial medical connectors have distinct names?
Connector naming is not included in the ISO 80369 series of standards. However, GEDSA and members of the ISO working group have proposed the name “NRFit” for connectors used in neuraxial and major regional applications, in compliance with the ISO 80369-6 standard.
Why is the industry adopting the new ISO neuraxial connector?
The Luer connector, though effective, has led to dangerous misconnections, such as delivering intravenous drugs via a neuraxial route. The ISO 80369-6 connector prevents wrong-route delivery of medications like bupivacaine and improves overall patient safety by making it impossible to connect neuraxial devices to devices for other routes of delivery.
What are the implications of these new standards?
Unique connector designs reduce the risk of wrong-route administration of fluids and medications. For healthcare providers, this change may require adjustments in sourcing supplies and assembling procedure packs. Pharmacy departments will need to ensure proper stocks of new syringes and other equipment for intrathecal and epidural medication preparation.
What connectors are affected by the ISO 80369-6 (NRFit) connector standard?
ISO 80369-6 covers medical devices that administer medication to or take samples from neuraxial sites, such as the spinal canal, and major peripheral nerve blocks. It also includes continuous wound infiltration and cerebrospinal fluid pressure monitoring or removal.
Which devices are affected by the ISO 80369-6 standard?
Neuraxial devices and those used for major regional anaesthesia that currently use Luer connectors will gradually incorporate the new ISO 80369-6 connectors over the next few years.
Who developed the proposed new ISO 80369-6 connector design?
The ISO 80369-6 connector design was developed by ISO with contributions from clinicians, regulators, and industry. It has undergone extensive testing, including misconnection tests and human factors assessments, to ensure its safety.
Why should we adopt the new 80369-6 connector?
The ISO 80369-6 connector enhances patient safety by reducing the risk of misconnections between neuraxial and non-neuraxial systems. In some countries, legal systems require healthcare providers to use connectors that meet these standards to avoid legal liability in the event of wrong-route incidents.
What makes the new ISO 80369-6 connector different from the current Luer system?
The new ISO 80369-6 connector looks like a Luer connector, but is about 20% smaller and has a unique design that reduces the risk of cross connection with other connectors developed under the same series of standards.
When will the new ISO 80369-6 connector be available?
Neuraxial devices with the ISO 80369-6 connector are expected to be available in markets like the UK, US, Canada, and others from 2017 onwards, subject to regulatory approvals and manufacturer readiness.
How will the new ISO 80369-6 connector be introduced into my healthcare organisation?
The introduction of the new connector will depend on local regulations. In some countries, it will be led by government or healthcare organisations. Healthcare facilities can work with manufacturers to plan a smooth transition from Luer to ISO 80369-6 devices. The global industry group has agreed to use a common brand name, NRFit, to help with this transition.
If we use Neuraxial parts from another manufacturer's set, how will we know the products will work together?
As long as the connectors comply with the ISO standard, they will connect safely and effectively. Many devices will be marked with the NRFit label or ISO 80369-6 standard number. Some neuraxial components may also feature yellow colouring for ease of identification.
Is it mandatory to transition to the new ISO 80369-6 connector?
This depends on the jurisdiction. For example, California mandated the transition by January 1, 2017. In the UK, the NHS is planning to adopt the standard within six months of the California deadline. Other regions may follow, depending on local regulations.
When will the neuraxial devices with current connectors be discontinued?
The discontinuation of devices is determined by manufacturers. Luer-based neuraxial devices will be phased out, but some long needles with Luer connectors will still be used for non-neuraxial procedures, such as amniocentesis. You should consult suppliers to confirm their plans for these devices.
Will there be new item numbers or SKUs for the new 80369-6 sets?
New item numbers are at the discretion of manufacturers. In some cases, manufacturers may use product codes that make it easy to distinguish between Luer and ISO 80369-6 devices.
If applicable, when will the new item numbers be available, and how will we know when to order them?
Manufacturers will communicate these changes directly to healthcare facilities. Stay in touch with your supplier to keep track of the new NRFit device numbers and ordering timelines.
Are these the final design for these connectors?
The design of ISO 80369-6 connectors has been finalised and published in 2016. This standard is considered final unless future amendments or revisions are made, which is typical for ISO standards.
Will these new 80369-6 connectors fit existing Luer devices?
No. The ISO 80369-6 connector is deliberately designed not to be compatible with Luer connectors to prevent dangerous misconnections between systems.
How will these changes be regulated?
Regulation will depend on the country and region. Some, like the US (through the FDA), UK, and others, have already set deadlines for adoption. Other areas may follow based on legal or clinical guidelines.
How should we prepare for this change in connectors?
Healthcare facilities should engage with manufacturers to stay updated on the availability of ISO 80369-6 compliant products. Training staff, adjusting supply chains, and ensuring the availability of the new equipment is essential for a smooth transition.
Where can I find more information on the ISO 80369-6 connector?
For more detailed information, consult your medical device supplier or manufacturer, or visit the Global Enteral Device Supplier Association (GEDSA) website for the latest updates on the implementation of ISO 80369-6 standards.
Still need more information?
Resources
Find everything you need to get started with NRFit in one place. Our resources include easy-to-follow guides, training materials, product details, and safety information to help you use NRFit safely and effectively.
At GBUK Group we value your feedback
We are committed to providing you with the best possible experience. If you have any questions, comments, or concerns, please do not hesitate to contact us.
Our customer service team is available to assist you with any inquiries you may have. You can reach us via phone, email, or by filling out the contact form. We appreciate your business and look forward to hearing from you!
Call us
Our Location
Contact Us